
A Covid-19 vaccine from Oxford University and AstraZeneca has been approved for use in the UK, paving the way for mass rollout.
The jab, which has been described as a “game changer”, was given the green light by the Medicines and Healthcare products Regulatory Agency (MHRA).
Health Secretary Matt Hancock said the approval is “fantastic news” and confirmed the rollout will begin on January 4, including to care homes.
He told Sky News: “I am now, with this approval this morning, highly confident that we can get enough vulnerable people vaccinated by the spring that we can now see the route out of this pandemic.”
He said there would be a difficult few weeks ahead “but we also know that there is a route out of this”.
He added: “The vaccine provides that route out. We have all just got to hold our nerve over the weeks to come.”
Today’s approval of the @UniofOxford / @AstraZeneca #coronavirus vaccine is a great day for British science supported by the UK government & NHS.
THANK YOU to all those involved
1/2 pic.twitter.com/uvA7g2FT1M
— Matt Hancock (@MattHancock) December 30, 2020
Prime Minister Boris Johnson tweeted: “It is truly fantastic news – and a triumph for British science – that the @UniofOxford/@AstraZeneca vaccine has been approved for use.
“We will now move to vaccinate as many people as quickly as possible.”
The news comes amid increasing strain on hospitals in England, where the number of Covid-19 patients is the highest it has been during the pandemic.
Mr Hancock is due to announce any changes to tier areas in a statement to the Commons on Wednesday.
“We are facing a very significant challenge in the NHS right now,” he told Sky News.
“There has been a significant rise in the number of cases – the highest number of cases recorded yesterday, 53,000 cases.
“We are going to have to take further action.”
 A researcher in a laboratory at the Jenner Institute, working on the coronavirus vaccine developed by AstraZeneca and Oxford University (John Cairns/University of Oxford/PA)
A researcher in a laboratory at the Jenner Institute, working on the coronavirus vaccine developed by AstraZeneca and Oxford University (John Cairns/University of Oxford/PA)
The UK has ordered 100 million doses of the Oxford University vaccine – enough to vaccinate 50 million people.
Mr Hancock said the plan is to vaccinate all vulnerable groups first but that eventually all adults, including the under-50s, will be offered a jab.
England’s chief medical officer, Professor Chris Whitty, said the jab is “safe and effective”, adding: “It is very good news that the independent regulator has now authorised for use the Oxford/AstraZeneca vaccine.”
The Joint Committee on Vaccination and Immunisation (JCVI), which advises ministers, will publish its latest guidance on who should receive the vaccine and in which order later.
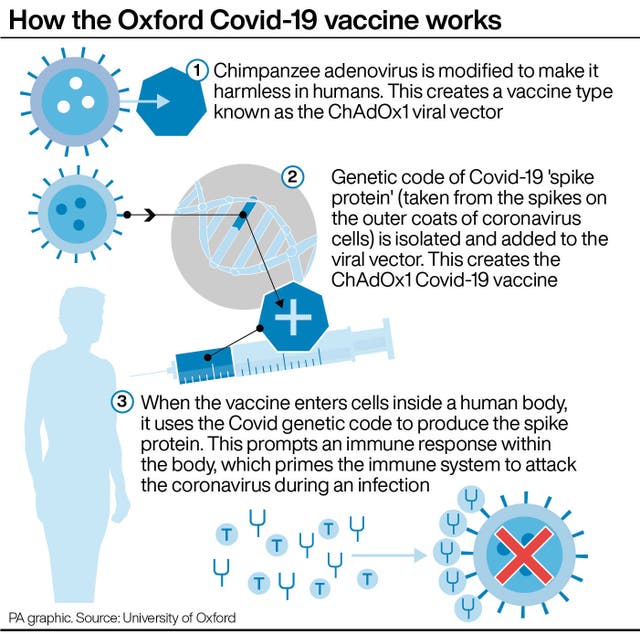 (PA Graphics)
(PA Graphics)
Data published in The Lancet medical journal in early December showed the vaccine was 62% effective in preventing Covid-19 among a group of 4,440 people given two standard doses of the vaccine when compared with 4,455 people given a placebo drug.
Of 1,367 people given a half first dose of the vaccine followed by a full second dose, there was 90% protection against Covid-19 when compared with a control group of 1,374 people.
The MHRA has authorised two full doses of the vaccine to be given to people.
In the vaccine trial, 10 people given the placebo dummy drug were admitted to hospital with coronavirus, including two with severe Covid which resulted in one death.
But among those receiving the vaccine, there were no hospital admissions or severe cases.
People receiving the Oxford vaccine or the one from Pfizer/BioNTech, which is also being rolled out, will now receive their first dose of the vaccine followed by a second dose up to 12 weeks later.
The aim is to give as many people as possible a first dose of a Covid-19 vaccine.
Mr Hancock said: “This is important because it means that we can get the first dose into more people more quickly and they can get the protection the first dose gives you.
“The scientists and the regulators have looked at the data and found that you get what they call ‘very effective protection’ from the first dose.
“The second dose is still important – especially for the long-term protection – but it does mean that we will be able to vaccinate more people more quickly than we previously could.”
In 2020, teams across AstraZeneca have risen to the challenges #COVID19 has posed to global health. Today’s advancement is a significant step forward in the fight against this pandemic. #WhatScienceCanDo pic.twitter.com/z8LvuFFMhS
— AstraZeneca (@AstraZeneca) December 30, 2020
AstraZeneca chief executive Pascal Soriot said: “Today is an important day for millions of people in the UK who will get access to this new vaccine.
“It has been shown to be effective, well-tolerated, simple to administer and is supplied by AstraZeneca at no profit.”
Professor Andrew Pollard, director of the Oxford Vaccine Group, who led the clinical trial, said it is “a landmark moment”.
But he said the impact of vaccines is “about getting them into people’s arms, stopping the virus from causing severe disease and hospitalisation, which we know that all of the vaccines can do very efficiently.”
He added: “There’s good protection with the first dose, up until the time of the second dose.
“And then the second dose is really important because we think that will be critical for the durability of the immune response that continues after that and hopefully will help us prevent further waves of disease once we have a large segment of the population vaccinated.”
During the Oxford vaccine trial, the half-dose followed by a full-dose regime came about as a result of an accident.
However, the MHRA was made aware of what happened and clinical trials for the vaccine were allowed to continue.
In an interview with the Sunday Times, Mr Soriot suggested that further data submitted to the regulator showed the vaccine could match the 95% efficacy achieved by the Pfizer/BioNTech and Moderna vaccines.
“We think we have figured out the winning formula and how to get efficacy that, after two doses, is up there with everybody else,” he said.
Shadow health secretary Jonathan Ashworth said the vaccine approval is “good news”, tweeting: “Now let’s go hell for leather to get jabs rolled out.”
The Oxford vaccine can be stored in a standard fridge, unlike the Pfizer/BioNTech jab, which needs cold storage of around minus 70C.
This means the Oxford vaccine is easier to roll out to places such as care homes and GP surgeries.


Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules hereLast Updated:
Report this comment Cancel